Cold Storage Platelets for all adult bleeding patients at Salem Hospital
08 Feb 2026Platelets issued based on availability
By: Andrew Judd, MD, Laboratory Medical Director and Jenny Clow, Lab Manager
Salem Hospital Blood Bank is now issuing cold storage platelets (CSP) for all actively bleeding adult patients, based on availability.
CSP are FDA approved for the treatment of active bleeding when conventional platelets are unavailable. They exist in an activated state, which means they are excellent at forming clots but have a shorter half-life in vivo. CSP have an extended shelf life of 14 days and will help ensure a more stable platelet inventory for bleeding patients.
This is our second test of change to eliminate blood product waste, which was anticipated to begin Monday, Feb. 9, but was moved up to due to the national platelet shortage. The first test of change was to include CSP in trauma packs, but we found that platelets were rarely transfused from trauma packs.
Test of Change details
- CSP will be sent in place of room temperature platelets as inventory allows.
- CSP will be sent in lavender coolers, if available, for adult patients who are actively bleeding.
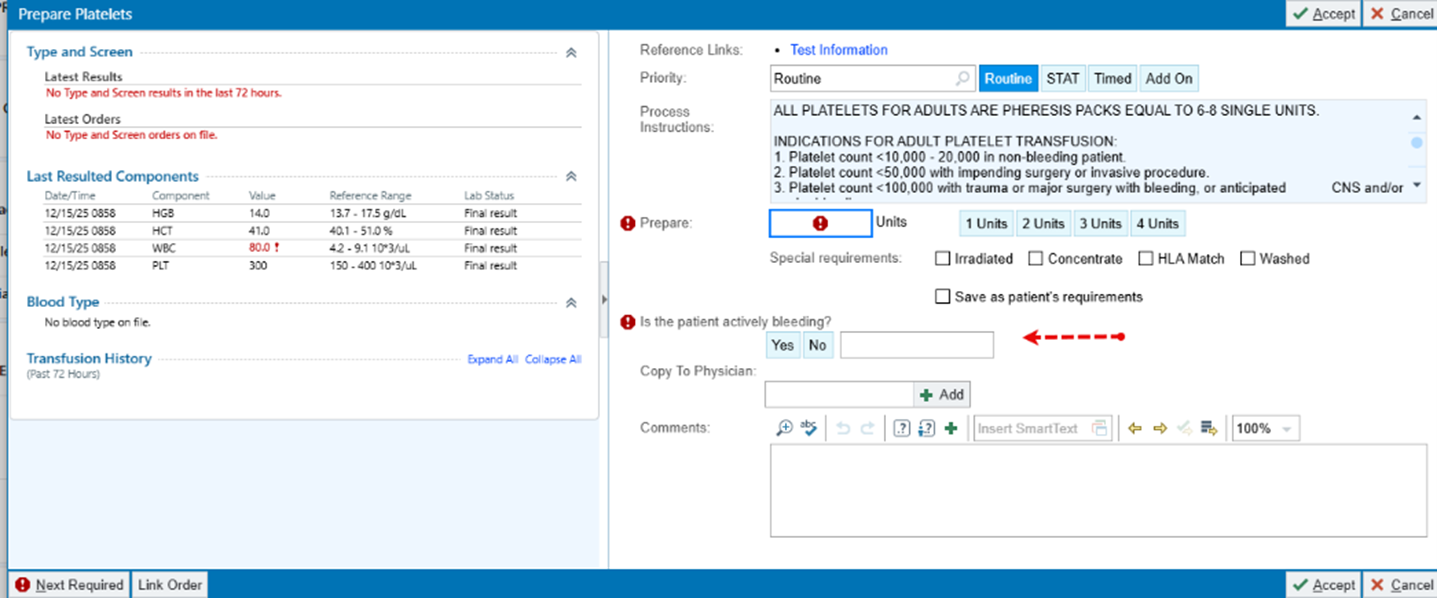
- To ensure FDA compliance the question “Is this patient actively bleeding?” has been added to the prepare platelets.
Want to learn more?
Watch the Dec. 2, 2025 Grand Rounds here (Salem Health intranet access required).
Questions?
Email Laboratory Medical Director Andrew Judd, MD, at andrew.judd@salemhealth.org or Laboratory Manager Jenny Clow at Jenny.Clow@salemhealth.org.