New test for the Hepatitis C virus starts June 2
18 May 2025Learn how to order, replaces current send-out test
By: Tish Gross, Salem Health Laboratories outreach supervisor
Salem Health Laboratories introduces a new test for the qualitative and quantitative detection of the Hepatitis C virus starting Monday, June 2.
This assay will aid in the diagnosis of active Hepatitis C (HCV) infections and managing HCV- infected patients undergoing HCV antiviral drug therapy. Detection of HCV RNA does not distinguish between acute and chronic states of infection. This assay can be used to measure HCV RNA levels periodically before, during and after treatment to determine sustained virological response (SVR) or non-sustained virological response (NSVR). The results of the Aptima HCV Quant Dx assay must be interpreted within the context of all relevant clinical and laboratory findings.
Note: The assay is not approved for use as a screening test for the presence of HCV RNA in blood or blood products.
This test will replace the current send out test “Hepatitis C Virus (HCV) by Quantitative NAAT,” Test code: XHCVN.
Specimen collection
Blood should be collected in Lavender (EDTA), ACD, Plasma Preparation Tube (PPT), Serum Tube or Serum Separator Tube (SST).
Specimen acceptability requirements and appropriate transport
- Sample type(s): Serum or plasma (collected using the containers listed above)
- Volume: 1.5 mL
- Transport: Refrigerated
- Sample storage/stability:
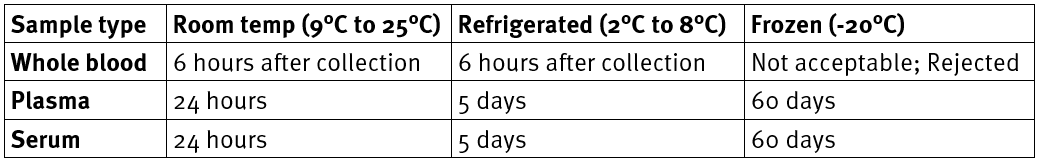
Specimen rejection criteria
- Blood not collected in the tubes listed under “Specimen collection.”
- Blood, serum or plasma not stored at the specified storage conditions listed under “Sample storage/stability.”
- Serum or plasma that has not been separated from red blood cells within 6 hours of specimen collection.
Specimen preparation
- Whole blood: Serum or plasma must be centrifuged and separated from red blood cells within 6 hours of collection.
- Serum: Allow clot to form in tube before further processing.
- Serum and plasma: Do not store in primary collection tubes for more than 6 hours after collection. Serum and plasma should be aliquoted to a secondary tube for long-term storage.
- Do not freeze samples in EDTA, ACD or serum primary collection tubes. Plasma and/or serum need to be transferred to a secondary tube prior to being frozen.
Specimen retention
- Five days
How to order

Test methodology
- Real-Time Transcription-Mediated Amplification (TMA)
Reference range/test location and turn-around-time (TAT)
- Hepatitis C (HCV): Not Detected
- Test Location: Salem Health Laboratory – State Street – Molecular
- Days Performed: Monday, Thursday and Saturday
- Reported: 1 to 3 Days
Testing questions?
Contact the Molecular/Microbiology Department at 503-814-1662. If you are unable to reach someone in the Molecular Department, please call Client Services at 503-814-5227.